Virtual Event
Evidence-Driven Approaches to Preventing Firearm Deaths
A Conversation with Andrew Morral, PhD, Joseph Richardson, Jr., PhD, and Ali Rowhani-Rahbar, PhD, MD, MPH, moderated by Therese Richmond, PhD, MSN
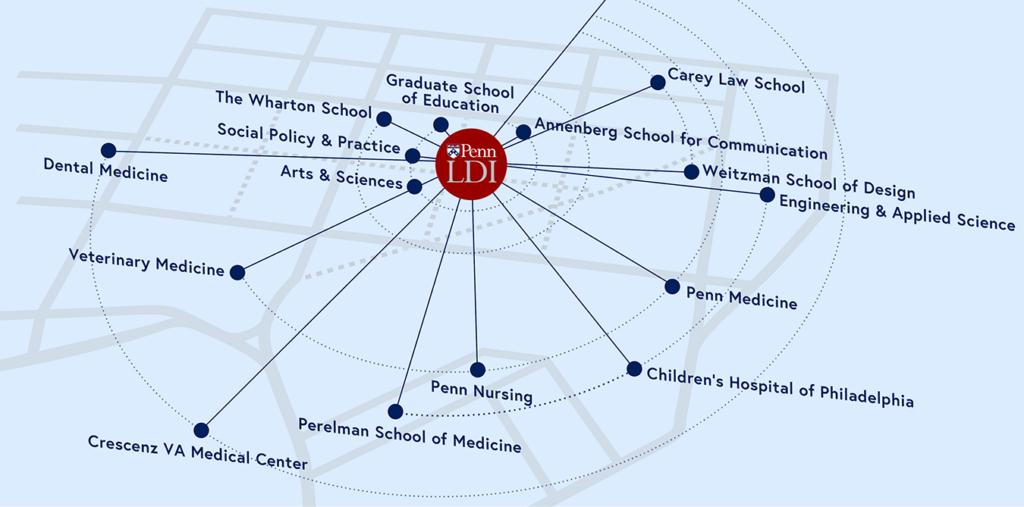
As Penn’s hub for health care delivery, health policy, and population health, we connect and amplify over 500 Fellows across the University, and train the next generation of researchers.
Virtual Event
A Conversation with Andrew Morral, PhD, Joseph Richardson, Jr., PhD, and Ali Rowhani-Rahbar, PhD, MD, MPH, moderated by Therese Richmond, PhD, MSN

$142 Million
In Research Grants Given Annually to Senior Fellows
141,957
Senior Fellow Journal Citations in 2018–2022
9,721
Articles Published by Senior Fellows in 2022